
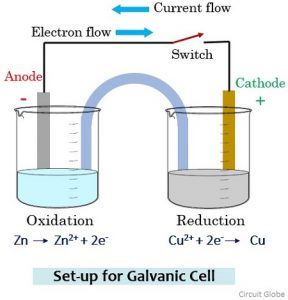
In the cell below, Zincis used for the electrode on the left (the Anode) in contact with a solutionof Zinc (II) ions, possibly a solution of ZincNitrate.

In most batteries, there are different materials atthe two electrodes, such that they want to react with one material beingoxidized and the other being reduced.

The heat released isclosely related to the standard enthalpy change ( delta- H° )of the reaction. Most of theenergy of the reaction is released as heat. If the reactive components ofan electrochemical cell are placed in contact with each other, they willreact by direct transfer of electrons ( anoxidation - reduction reaction ) and thereis no way to harness this energy to do electrical work. The batteryoperates through electrochemical reactions called oxidation and reduction.These reactions involve the exchange of electrons between chemical species.If a chemical species loses one or more electrons, this is called oxidation.The opposite process, the gain of electrons, is called reduction. A battery consists of one or more electrochemical cells.Each cell contains two metal electrodes and at least one electrolyte solution(a solution containing ions that can conduct electricity).


 0 kommentar(er)
0 kommentar(er)
